ICU Management & Practice, Volume 24 - Issue 3, 2024
Pain is defined as an unpleasant sensory and emotional experience. Among patients admitted to the Intensive Care Unit (ICU), severe untreated pain is associated with an increase in mortality, length of hospital stays and worsening in everyday quality of life after hospital discharge. Pain in critically ill patients is more difficult to monitor and manage due to several factors, such as the presence of patients unable to communicate and severe clinical alterations limiting the use of analgesic drugs or the application of analgesic locoregional techniques. We present an extensive narrative review on ICU pain treatment, focusing on tools used to detect this condition and multimodal strategies adopted to reach adequate analgesia.
Introduction
The Association for the Study of Pain (IASP) defines pain as "an unpleasant sensory and emotional experience associated with actual or potential tissue injury or described in terms of such damage'' (Pain 1979).
International PATHOS study (Benhamou et al. 2008), involving 746 European hospitals, highlighted the suboptimal management of postoperative analgesia, supporting the need for improving pain treatment in surgical European wards. This assessment becomes even more critical when considering intensive care unit (ICU) patients. According to recent reports, more than 5 million patients are admitted to ICU in the United States every year. Pain at rest is detected in over half of them, and this number increases to 80% when considering procedural pain (Devlin et al. 2018).
Analgesia in critically ill patients can be very difficult to manage due to several factors, including a limited or a total loss in the patient's ability to communicate, severe emotional distress and important biological alterations that can alter the pharmacokinetic and pharmacodynamic profile of the analgesic drug, restricting their use. Untreated ICU pain is associated with an increase in death, in-hospital delirium, and the development of chronic pain, with a negative impact on the quality of life after hospital discharge (Yamashita et al. 2017). We present an extensive narrative review on ICU pain treatment, focusing on tools used to detect this condition and multimodal strategies adopted to reach adequate analgesia.
Pain Monitoring
Pain is a negative experience for patients in the ICU, where they often undergo invasive and non-invasive procedures (turning, endotracheal suctioning, wound care, central venous catheter and arterial line insertion) (Puntillo et al. 2014). Furthermore, they may experience pain from surgical wounds and underlying conditions. Pain monitoring is important for reducing adverse outcomes such as ICU length of stay and duration of mechanical ventilation (Payen et al. 2007), delirium, post-traumatic stress disorder (PTSD) and increased mortality (Kastrup et al. 2009; Payen et al. 2009). However, assessing pain in critically ill patients can be challenging due to factors such as sedation, mechanical ventilation, and altered consciousness; indeed, all these factors prevent patients from verbally communicating their pain (Ahlers et al. 2008).
According to a 2021 review on pain monitoring in the ICU, pain assessment should take place on admission to the ICU, adopting, even before assessment scales, mnemonic tools useful for focusing on certain aspects of pain. The PQRSTUV mnemonic (Nordness et al. 2021) is frequently used, and it is based on these items:
- Provocative/Palliative factors: Pain cause; pain-relieving strategies;
- Quality: Pain sensation;
- Region: Pain location;
- Severity: Pain intensity;
- Time: Pain duration or temporality;
- Understand: Previous pain experience and known problems;
- Values and preferences for pain treatment.
Pain assessment in the ICU relies on subjective measures such as self-reporting in conscious patients or observational scales for unconscious patients (Devlin et al. 2018). These methods are very useful, but they are limited by several factors, such as subjectivity in evaluation and the need for patient cooperation. There is a high risk of not capturing fluctuations in pain levels over time.
Rating scales commonly used in intensive care are divisible into:
- Unidimensional scales, which measure only intensity, include the Numeric Rating Scale (NRS), Visual Analogue Scale (VAS) and Verbal Rating/Descriptive Scale (VRS/VDS) (Chanques et al. 2022).
- Behavioural scales include Behavioural Pain Scale (BPS), Critical Care Pain Observational Tool (CPOT), Non-Verbal Pain Scale (NVPS) and Pain Assessment in Advanced Dementia (PAINAD).
Numeric Rating Scale
The Numeric Rating Scale (NRS) provides a simple and standardised method for quantifying pain intensity, allowing healthcare providers to assess and monitor pain in ICU patients. This unidimensional scale is a self-reporting scale where individuals rate their pain intensity by selecting a number from 0 to 10 verbally (Puntillo et al. 1997) with:
- 0 = no pain
- 1-3 = mild pain
- 4-6 = moderate pain
- 7-10 = severe pain
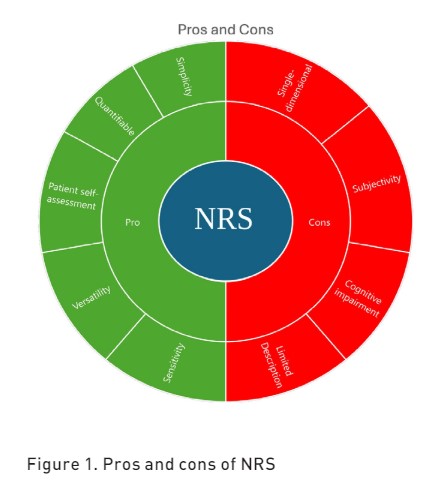
The NRS has a maximum acceptable score of 3 (Hamill-Ruth et al. 1999) and can be used across different populations, including adults, children older than eight years and elders, due to its simplicity and ability to provide a quantitative measure of pain intensity (Sessler et al. 2008). The NRS is also the most used scale in cancer patients (Oldenmenger et al. 2017). In the ICU, NRS can also be administered to patients unable to communicate through visual aids (NRS-V), preferably in a large format, making it a usable scale even in lightly sedated patients (Richmond Agitation-Sedation scale (RASS) score greater than -2) (G. Chanques et al. 2022). The pros and cons of NRS are reported in Figure 1.
Visual Analogue Scale
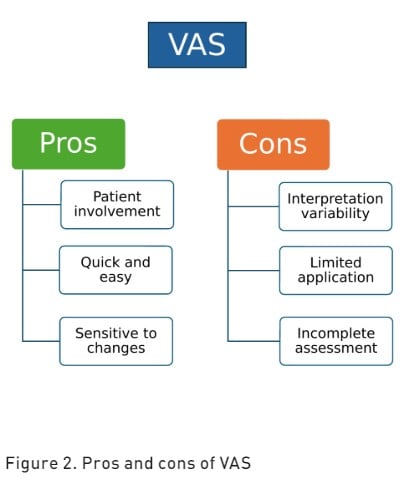
Visual Analogue Scale (VAS) is a subjective pain assessment tool that measures both pain intensity and the extent of pain relief (Karcioglu et al. 2018). The VAS is represented as a continuous horizontal (HVAS) or vertical (VVAS) line of 100 millimetres in length with a cursor anchored by verbal descriptors at each end. This pain rating scale has a maximum acceptable score of 30 mm (Ahlers et al. 2008). Patients are asked to mark a point on the line that corresponds to their current level of pain intensity, with one end representing "no pain" and the other end representing "worst pain imaginable" (Jensen et al. 1986). The distance from the "no pain" end to the patient's mark is then measured to quantify pain intensity. The pros and cons of VAS are reported in Figure 2.
Verbal Rating/Descriptor Scale
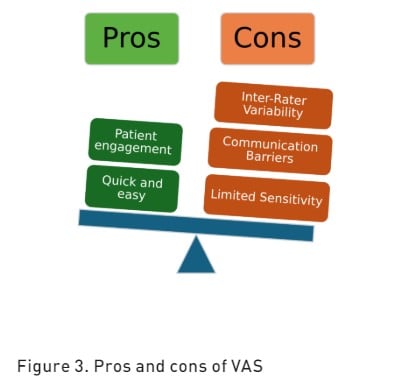
Verbal Rating/Descriptor Scale (VRS/VDS) is a validated pain assessment tool that relies on verbal communication to quantify the intensity of perceived pain (Williamson et al. 2005). This evaluation typically consists of a series of descriptive terms that represent different levels of pain severity. Commonly utilised descriptors include "no pain," "mild pain," "moderate pain," and "severe pain" (Karcioglu et al. 2018). The VRS was the most successful pain scale used in patients with cognitive impairment (Shimoji 2020). The pros and cons of VRS are reported in Figure 3.
Several studies have concluded that the most accurate assessment of a patient’s pain is a patient’s self-report; the recommended and most widely used scales are NRS and VAS, but these scales can struggle with the reduced consciousness and cognitive impairments often found in ICU patients. However, according to current guidelines, the easiest pain rating scale to use in the ICU, with the highest success rate, and with the best sensitivity and negative predictive value is the NRS (Devlin et al. 2018).
Behavioural Scales
The most cited and utilised scales in intensive care therapies are the Critical-Care Pain Observation Tool (CPOT) and the Behavioural Pain Scale (BPS).
CPOT (Gélinas et al. 2004) is an observational scale that analyses four items:
- facial expression
- body movements
- upper limb muscle tension
- ventilator compliance
Each item is given two points ranging from 2 (no pain) to 8 points (maximum pain). The process of evaluating pain in patients involves several steps:
- establishing a baseline CPOT value while the patient is at rest
- closely monitoring patients’ responses during nociceptive procedures
- assessing pain levels before and after administering analgesic agents
- assigning the highest observed CPOT score during evaluation
- scoring each behaviour component of the CPOT, with special attention to muscle tension
This comprehensive approach can ensure a thorough assessment of pain in ICU clinical settings.
BPS (Li et al. 2008) is an observational scale that considers three items:
- facial expression
- upper limb movement
- ventilator compliance
Each item is assigned a score from 1 (no response) to 4 (full response).
When compared with the CPOT scale, the BPS scale showed greater variability in pain score measurement during non-painful procedures like mouthwash and oral care (Gomarverdi et al. 2019). The main limitation of BPS is its consideration of upper limb movement as an integral part of the nociceptive reflex, when in many manoeuvres, this action can be linked only to a non-nociceptive reflex stimulus (Rijkenberg et al. 2017).
Pain Assessment in Advanced Dementia
Another pain assessment scale is Pain Assessment in Advanced Dementia (PAINAD), a behaviour-observation tool developed for patients with advanced dementia who lack verbal communication abilities to express pain (Warden et al. 2003). The PAINAD assesses pain through five specific indicators: breathing, vocalisation, facial expression, body language, and consolability. Scores range from 0 to 10, with higher scores indicating more severe pain; in recent years, this tool has also been utilised for sedated or non-verbally expressive patients in ICU.
Nonverbal Adult Pain Assessment Scale
Incorporating patient parameter assessment alongside the previously described items used in CPOT and BPS, we can introduce the Nonverbal Adult Pain Assessment Scale (NVPS) (Azevedo-Santos et al. 2018). The updated version considers five responses:
- facial expression
- activity (movement) guarding
- baseline Respiratory Rate (RR)/SpO2, ventilator compliance
- physiological parameters (vital signs including blood pressure (BP), heart rate (HR), resting rate (RR).
Each parameter is rated on a scale from 0 to 2, with a total score ranging from 0 (showing no pain) to 10 (indicating maximum pain), with a cut-off of >4 indicating significant pain. The possible misinterpretation of vital signs nonspecific to pain is the main limitation of this scale.
Prospects
The use of innovative technologies in intensive care could improve the pain management of critically ill patients. Some of these technologies such as NOL, ANI, Pupillometer and qNOX can be used for pain monitoring during general anaesthesia in surgery; however, currently, none of these tools has been widely adopted in the ICU because of the lack of strong supporting evidence.
Analgesia Nociception Index
The Analgesia Nociception Index(ANI) is a pain assessment tool that evaluates a single physiological parameter, specifically, the high-frequency spectrum of heart rate variability (HRV) induced by each respiratory cycle from ECG monitoring of the patient. Data are collected from two electrodes placed on the sternum and axillary midline and analysed by software that provides an index indicating the balance between nociception (low parasympathetic modulation) and analgesia (high parasympathetic modulation). The values provided range from 100 (low level of pain and stress) to 0 (high level of pain and stress). The optimal value that should be obtained during general anaesthesia is between 50 and 70; if less than 50, pain is undertreated; if more than 70, the pain has been overtreated (Shiva et al. 2021). A study on the use of ANI in the ICU to assess pain in critically ill patients during non-painful and painful procedures showed that Mean-ANI (ANIm mean-ANI which is calculated over the previous 4 minutes) is not suitable for ICU patients but Istant-ANI (ANIi calculated in a short period of about 1 minute) is useful for identifying pain in the ICU setting with a negative predictive value of 90% and higher sensitivity in detecting pain during minor procedures (dressing change) than BPS (Chanques et al. 2017). However, ANI can be influenced by several factors, such as age, obesity, disease severity, mechanical ventilation, and anxiety and stress, leading to overestimation or underestimation of pain.
Nociception Level Index
The Nociception Level Index (NOL)is a multi-parameter pain monitoring instrument that provides via a probe placed on a finger a value between 0 and 100, shown on the Pain Monitoring Device monitor (PMD2000 Medasense Biometrics Ltd., Ramat Gan, Israel.) The finger probe includes a sensor to record heart rate (HR), HRV, photoplethysmography wave amplitude, skin conductance level, number of skin conductance fluctuations, skin temperature, and their time derivatives. All these parameters are integrated with a non-linear Random Forest regression technique to provide the NOL index. A value <25 is indicative of pain (Shiva et al. 2021). A study conducted in ICUs to evaluate the NOL's ability to discriminate between nociceptive and non-nociceptive stimuli demonstrated the possibility of exploiting this device to detect pain in critically ill patients, finding confirmation in the parallel measurement made with the CPOT (Shiva et al. 2020). However, there are still few studies on NOL in the ICU to draw any conclusions about the application of this device in this clinical scenario.
qNOX
The qNOX score (ranging from 0 to 99) is a dimensionless proprietary score based on EEG (electroencephalography) and EMG (electromyography) measurements. It is designed to gauge the likelihood of a patient's movement response to a noxious stimulus. The manufacturer suggests that a qNOX score below 40 shows a very low likelihood of a response to a painful stimulus; conversely, a low likelihood with a score between 40 and 60 and a higher likelihood when the score is above 60. The independence of this device from various potential confounders, such as vasoactive drugs, makes this tool conceptually appealing. Boselli et al. (2023) evaluated in a small retrospective study the utility of the qNOX score, particularly concerning its efficacy in discerning responses to noxious stimuli (tracheal suction) among patients under profound sedation and neuromuscular blockade within the ICU setting.
Pupillometry
Pupillometry appears as a valuable adjunctive tool for pain assessment in critically ill patients admitted to the ICU. Recent investigations underscored its reliability, particularly among sedated individuals. Audicana et al. (2023) elucidated the significance of pupillometry in patients exhibiting a RASS score ranging from -4 to -1. They observed that pupillary diameter responses (PDR) offered superior discrimination of heightened pain responses compared to BPS. Moreover, in patients with a more profound sedation level (RASS score 5) (Lukaszewica et al. 2015), pupillary changes were effective in assessing analgesic depth and predicting the presence of pain during invasive procedures performed in the ICU.
Pain Management
The primary goal of pain management in the ICU is to maximise patient comfort. Failing to ensure this important clinical outcome can lead to negative physiological effects, development of chronic pain conditions and increased anxiety and agitation (Lewis et al. 1994; Battle et al. 2013). A thorough evaluation of pain should be paired with a multi-modal treatment strategy, adopting pharmacological and non-pharmacological techniques for alleviating pain (Nordness et al. 2021).
Pharmacological Strategies
For critically ill patients, managing pain typically involves pharmacological approaches, commonly based on the administration of opioids (Devlin et al. 2018; Posa et al. 2020). However, extended use of opioids can result in increased drug tolerance, necessitating larger doses for the same pain relief. Prolonged administration at increasing dosages can also lead to physical dependence and withdrawal symptoms when reducing opioid use. Moreover, such prolonged opioid consumption may contribute to the onset of chronic pain and induced hyperalgesia (Chu et al. 2008; Puntillo et al. 2016). To counteract these risks, an appropriate use of opioids, with an adequate plan of opioid rotation and coadministration of nonopioid analgesics is required. A tiered pain management strategy is also recommended, analogous to the approach the World Health Organization suggests for cancer patient care (Ventafridda et al. 1985). Current guidelines also recommend the strategy of analgosedation, which prioritises pain management before starting sedation therapy, using sedation only if necessary (Pun et al. 2019).
Routes Of Administration
The reasons for ICU admissions are diverse, primarily categorised into surgical and medical patients, each presenting with various states of dysfunction, including cerebral, cardiovascular, renal, and respiratory issues. The administration of drugs must always consider potential risks, with adjustments in route and dosage tailored to the individual patient's needs. Intravenous (IV) administration is preferred over intramuscular or subcutaneous routes due to the unpredictable bioavailability associated with the last two methods (Devlin et al. 2018; Chou et al. 2016). However, alternative methods like regional analgesia or patient-controlled analgesia (PCA) may be selected depending on the patient's state of consciousness and the nature of their pain, such as postoperative discomfort (Levy et al. 2011). These choices are guided by the principle of multimodal analgesia, with the goal of reducing opioid consumption (Wick et al. 2017).
Opioid Analgesics
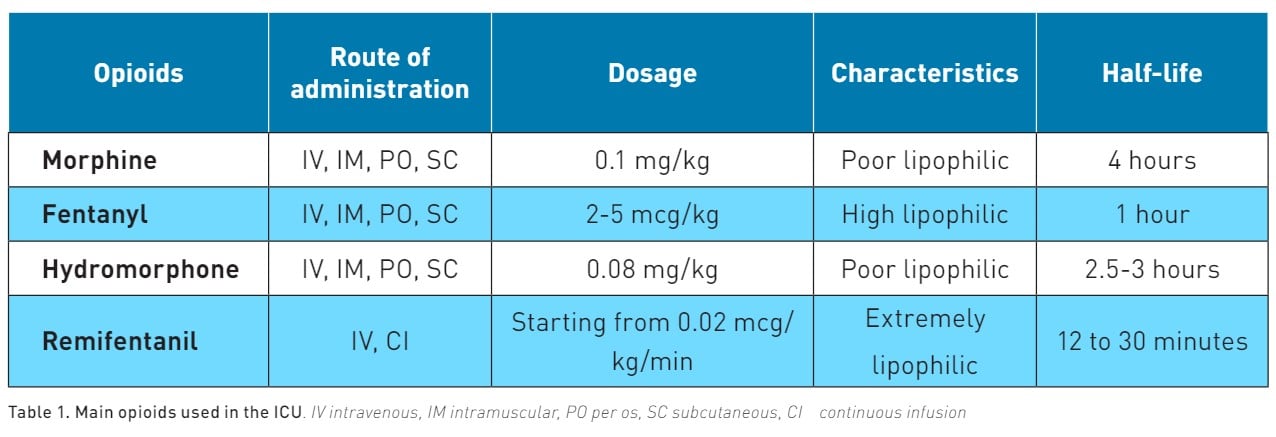
Opioids alleviate pain by acting on specific areas of the brain (cortex, thalamus, hypothalamus, locus coeruleus, amygdala, and periaqueductal grey matter) as well as the spinal cord and the membranes of peripheral nerves (Martyn et al. 2019). Opioid receptors, like Mu and Delta receptors (MORs and DORs), are both Gαi/o-coupled GPCRs and are activated by opioids. This activation leads to a reduction in neuronal activity and synaptic transmission. The main characteristics of opioids used in ICU are reported in Table 1.
Morphine is poorly lipophilic, and like all opioids, this drug undergoes primary metabolism in the liver, with metabolites being expelled through urine. Morphine has an onset of action of 5 to 10 minutes with a half-life of 4 hours and several active metabolites that can accumulate in the case of renal failure. To prevent this effect, the maintenance dose of morphine must be reduced by 50% in this category of patients (Aronoff et al. 2007). Morphine also stimulates the release of histamine, which can cause hypotensive events (Lambert et al. 2023). However, the release of histamine-induced by morphine does not cause bronchoconstriction (Eschenbacher et al. 1984).
Fentanyl is 600 times more lipid soluble compared to morphine. This characteristic enables fentanyl to have a rapid onset of action, approximately 1 minute, and a relatively short half-life ranging from 0.5 to 1 hour. Bolus administration could be useful in the management of procedural pain (Siffleet et al. 2007; Robleda et al. 2016). The metabolism of fentanyl occurs in the liver, leading to the production of inactive metabolites that are then excreted. This metabolic pathway makes fentanyl a more suitable option for patients with renal insufficiency (Davison et al. 2019). Despite its advantages, the use of fentanyl requires careful monitoring. Its administration can result in possible adverse effects, including respiratory depression (Dahan et al. 2010).
Hydromorphone is considered 5 to 10 times more potent than morphine (Felden et al. 2011). Its onset of action occurs within 15 to 30 minutes, and it has a half-life ranging from 2 to 3 hours. While it may require dose reduction in patients with renal impairment, hydromorphone is a beneficial option for dialysis patients since hydromorphone-3-glucuronide is removed during haemodialysis (Davison et al. 2008).
Remifentanil is an extremely lipophilic drug. This characteristic enables it to have a rapid onset of action, approximately 1 minute, and an extremely short half-life ranging from 12 to 30 minutes (Kapila et al. 1995). It is administered as a continuous infusion. Since remifentanil is metabolised by nonspecific esterase in the plasma without involving the liver or kidneys, no dose adjustment is required for patients with renal or hepatic insufficiency. This feature, combined with its rapid onset and offset, positions remifentanil as the preferred sedative-analgesic agent in ICU, allowing frequent neurological assessments and reducing the time to extubation (Dahaba et al. 2004; Breen et al. 2005).
Non-Opioid Analgesics
Acetaminophen's pain-relieving effect primarily occurs through the activation of descending serotonergic pathways. However, there is some discussion regarding the main mechanism of action, which is believed to involve the suppression of prostaglandin synthesis (Anderson et al. 2008). This drug is indicated in the treatment of fever and mild pain, and it should be used as an adjunct to opioids to reduce pain intensity and opioid consumption for pain management in critically ill adults (Devlin et al. 2018). The recommended dosing is 1g every 6 hours IV with a maximum dose of 4g. Acetaminophen’s dose adjustment should be applied for patients with mild to moderate hepatic insufficiency or body weight less than 50 kg, with no reduction for renal impairment. In the setting of ICU, a prospective observational study (Cantais et al. 2016) demonstrated a correlation between IV acetaminophen administration and development of hypotension in half of the treated patients, with the need of therapeutic intervention in one third of observed episodes.
Nonsteroidal Anti-Inflammatory Drugs
Nonsteroidal anti-inflammatorydrugs (NSAIDs) non-selectively inhibit cyclooxygenase, playing a key role in reducing inflammation. However, this mechanism also poses a risk of adverse events, particularly affecting the gastrointestinal tract and causing renal impairment.
Ketolorac- dosing 30 mg IV, maximum 120 mg per day for up to 5 days.
Ibuprofen - dosing 400-600 IV, maximum 3.2 g/day.
NSAIDs are indicated in the short-term treatment of moderate pain and as adjuncts in multimodal therapeutic regimens, with the goal of reducing opioid consumption.
Ketamine
Therecommended dosage for ketamine is 0.5 mg/kg bolus followed by a 1-2 mcg/kg/min infusion (Cook et al. 2020). This drug provides strong pain relief through its action of blocking N-methyl-D-aspartate (NMDA) receptors, thereby reducing the release of glutamate and attaching to sigma-opioid receptors (Nadeson et al. 2002). Low-dose ketamine is advocated as a supplementary treatment alongside opioid therapy, aimed at minimising opioid intake in adults who have undergone surgery and are admitted to the ICU. A recent meta-analysis showed that ketamine had better analgesic effects in the early treatment of acute pain, while morphine maintained more durable effects (Juan Guo et al. 2024).
Neuropathic Pain Medications
The use of neuropathic pain medications, in addition to opioids for neuropathic pain management, is recommended by the Society of Critical Care Medicine (SCCM) guidelines (Devlin et al. 2018).
Their analgesic effects are mainly due to blocking calcium channels which reduce the release of excitatory neurotransmitters, dampening pain-enhancing signals from the brain and reducing inflammation with a positive impact on the emotional aspects of pain (Chincholkar et al. 2018).
Gabapentin – dosing oral - initially 100mg 3 times per day - maintenance 900 to 3600mg per day in three different administrations.
Pregabalin– dosing oral: initially 75mg – maintenance 150 to 300 twice per day.
Carbamazepine– dosing oral: 200 to 400 mg/day in 2-4 divided doses – maintenance
600 to 800 mg/day in 2-4 divided doses. Maximum 1.2 g/day.
Non-Pharmacological Strategies
A recent review by Nordness et al. (2021) emphasises the significance of nonpharmacological strategies, underlining four key points that are also reflected in the SCCM guidelines (Devlin et al. 2018).
Non-pharmacological interventions include a range of practices, from massage and cold therapy to music/sound therapy and relaxation techniques. These methods aim not only to alleviate the physical aspects of pain but also to address the emotional and psychological components. For example, massage therapy can provide relief and comfort to patients, potentially reducing the need for higher doses of pain medications (Mitchinson et al. 2007). Similarly, music and sound therapy can offer a soothing and distracting influence, which may help in reducing pain perception (Jaber et al. 2007).
These strategies offer several benefits, including minimising the reliance on pharmacological interventions, which can have side effects and contribute to issues such as opioid dependence.
Employing such approaches can contribute to achieving better pain management outcomes, enhancing patient comfort and facilitating recovery. As the field continues to evolve, further research and integration of nonpharmacological pain management strategies will be essential in improving care for ICU patients.
Locoregional Techniques
Enhanced Recovery after Surgery (ERAS) protocols supported the use of multimodal strategies to manage postoperative pain, including not only pharmacological approaches but also locoregional techniques. Locoregional blocks are a fundamental part of most analgesic ERAS protocols for surgery (Mancel et al. 2021). Thoracic epidural catheter placement is the gold standard approach to manage severe abdominal and thoracic pain after laparotomic surgical procedures. This technique, although effective, may not be used in critically ill patients due to several factors, including the risk of serious central neuraxial infections, the presence of sepsis, haemodynamic instability, and haemostatic abnormalities. The diffusion of ultrasound-guided manoeuvres allowed the application of several locoregional techniques also in critically ill ICU patients. Fascial ultrasound-guided blocks were demonstrated to be safe and effective for pain management of the abdomen (Tranversus Abdominis Plane-TAP- block) (Niraj et al. 2009) and thorax (Erector Spinae Plane-ESP -block) (Gursoy et al. 2020).
However, all these blocks should be performed only by clinicians with a high level of experience. Future large randomised controlled trials are necessary to better define the role of locoregional procedures in achieving pain control among ICU patients.
Conclusion
Pain in ICU patients is a complex and multifactorial condition, very difficult to detect and manage. Inadequate ICU pain management is associated with an increase in mortality and morbidity during hospital stay and severe worsening in the everyday quality of life after discharge. Pain should be considered as important as other vital parameters, and analgesia should be managed as other life-support systems. The definition of institutional standardised protocols, including tools to detect and monitor pain and multimodal therapeutic analgesic strategies, should be mandatory in ICU patients' management.
Conflict of Interest
None.
References:
Ahlers SJ, van Gulik L, van der Veen AM et al. (2008) Comparison of different pain scoring systems in critically ill patients in a general ICU. Crit Care. 12(1):R15.
Aronoff GR, Bennett WM, Berns JS et al. (2007) Drug prescribing in renal failure: dosing guidelines for adults and children. 5th ed. American College of Physicians.
Azevedo-Santos IF, De Santana JM (2018) Pain measurement techniques: Spotlight on mechanically ventilated patients. J Pain Res. 11:2069-2980.
Battle CE, Lovett S, Hutchings H (2013) Chronic pain in survivors of critical illness: a retrospective analysis of incidence and risk factors. Crit Care. 17(3):R101.
Benhamou D, Berti M, Brodner G et al. (2008) Postoperative Analgesic THerapy Observational Survey (PATHOS): A practice pattern study in 7 Central/Southern European countries. Pain. 136(1-2):134-141.
Boselli E, Fatah A, Ledochowski S et al. (2023) Variations of qCON and qNOX during tracheal suction in ICU patients on sedation and curarization for SARS-CoV2 pneumonia: a retrospective study. J Clin Monit Comput. 37(4):1119-1121.
Breen D, Karabinis A, Malbrain M et al. (2005) Decreased duration of mechanical ventilation when comparing analgesia-based sedation using remifentanil with standard hypnotic-based sedation for up to 10 days in intensive care unit patients: a randomized trial. Crit Care. 9(3):R200-R210.
Cantais A, Schnell D, Vincent F et al. (2016) Acetaminophen-Induced Changes in Systemic Blood Pressure in Critically Ill Patients: Results of a Multicenter Cohort Study. Crit Care Med. 44(12):2192-2198.
Chanques G, Gelinas C (2022) Monitoring pain in the intensive care unit (ICU). Intensive Care Med. 48:1508-1511.
Chanques G, Tarri T, Ride A et al. (2017) Analgesia nociception index for the assessment of pain in critically ill patients: a diagnostic accuracy study. Br J Anaesth. 119(4):812-820.
Chincholkar M (2018) Analgesic mechanisms of gabapentinoids and effects in experimental pain models: a narrative review. Br J Anaesth. 120(6):1315-1334.
Chou R, Gordon DB, de Leon-Casasola OA et al. (2016) Management of postoperative pain: A clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists' Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 17(2):131-157. Dosage error in article text. J Pain. 17(4):508-10.
Chu LF, Angst MS, Clark D (2008) Opioid-induced hyperalgesia in humans: molecular mechanisms and clinical considerations. Clin J Pain. 24:479-496.
Cook M, Barton C (2020) Pain management in the unstable trauma patient. Curr Trauma Rep. 6:154–160.
Dahaba AA, Grabner T, Rehak PH et al. (2004) Remifentanil versus morphine analgesia and sedation for mechanically ventilated critically ill patients: a randomized double-blind study. Anesthesiology. 101(3):640–646.
Dahan A, Aarts L, Smith TW (2010) Incidence, reversal, and prevention of opioid-induced respiratory depression. Anesthesiology. 112(1):226-238.
Davison SN, Mayo PR (2008) Pain management in chronic kidney disease: the pharmacokinetics and pharmacodynamics of hydromorphone and hydromorphone-3-glucuronide in hemodialysis patients. J Opioid Manag. 4(6):335-344.
Devlin J, Skrobik Y, Gelinas C et al. (2018) Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 46(9):e825-e873.
Eschenbacher WL, Bethel RA, Boushey HA, Sheppard D (1984) Morphine sulfate inhibits bronchoconstriction in subjects with mild asthma whose responses are inhibited by atropine. Am Rev Respir Dis. 130(3):363-367.
Felden L, Walter C, Harder S et al. (2011) Comparative clinical effects of hydromorphone and morphine: a meta-analysis. Br J Anaesth. 107(3):319-328.
Gélinas C, Fortier M, Viens C et al. (2004) Pain assessment and management in critically ill intubated patients: a retrospective study. Am J Crit Care. 13(2):126-135.
Gomarverdi S, Sedighie L, Seifrabiei M et al. (2019) Comparison of two pain scales: Behavioral pain scale and critical-care pain observation tool during invasive and noninvasive procedures in intensive care unit-admitted patients. Iranian J Nurs Midwifery Res. 24(2):151-155.
Guo J, Zhao F, Bian J et al. (2024) Low-dose ketamine versus morphine in the treatment of acute pain in the emergency department: a meta-analysis of 15 randomized controlled trials. Am J Emerg Med. 42:140-149.
Gursoy C, Kuscu Y, Demirbilek SG (2020) Pain management for traumatic rib fractures with ESP block in ICU. J Coll Physicians Surg Pak. 30(3):318-320.
Hamill-Ruth RJ, Marohn ML (1999) Evaluation of pain in the critically ill patient. Crit Care Clin. 15(1):35-54.
Jaber S, Bahloul H, Guétin S et al. (2007) Effects of music therapy in intensive care unit without sedation weaning patients versus non-ventilated patients. Ann Fr Anesth Reanim. 26:30–38.
Jensen MP, Karoly P, Braver S (1986) The measurement of clinical pain intensity: a comparison of six methods. Pain. 27(1):117-126.
Kapila A, Glass PS, Jacobs JR et al. (1995) Measured context-sensitive half-times of remifentanil and alfentanil. Anesthesiology. 83(5):968-975.
Karcioglu O, Topacoglu H, Dikme O et al. (2018) A systematic review of the pain scales in adults: Which to use? Am J Emerg Med. 36(4):707-714.
Kastrup M, von Dossow V, Seeling M et al. (2009) Key performance indicators in intensive care medicine. A retrospective matched cohort study. J Int Med Res. 37(5):1267-1284.
Lambert DG (2023) Opioids and opioid receptors; understanding pharmacological mechanisms as a key to therapeutic advances and mitigation of the misuse crisis. BJA Open. 6:100141.
Levy BF, Scott MJ, Fawcett W et al. (2011) Randomised clinical trial of epidural, spinal or patient-controlled analgesia for patients undergoing laparoscopic colorectal surgery. Br J Surg. 98(8):1068-1078.
Lewis KS, Whipple JK, Michael KA, Quebbeman EJ (1994) Effect of analgesic treatment on the physiological consequences of acute pain. Am J Hosp Pharm. 51(12):1539-1554.
Li D, Puntillo K, Miaskowski C (2008) A review of objective pain measures for use with critical care adult patients unable to self-report. J Pain. 9(1):2-10.
López de Audícana-Jimenez de Aberasturi Y, Vallejo-De la Cueva A, Aretxabala-Cortajarena N et al. (2023) Pupillary dilation reflex and behavioural pain scale: Study of diagnostic test. Intensive Crit Care Nurs. 74:103332.
Lukaszewica AC, Dereu D, Gayat E et al. (2015) The relevance of pupillometry for evaluation of analgesia before noxious procedures in the intensive care unit. Anesth Analg. 120(6):1297-1300.
Mancel L, Van Loon K, Lopez AM (2021) Role of regional anesthesia in Enhanced Recovery after Surgery (ERAS) protocols. Curr Opin Anaesthesiol. 34(1):616-625.
Martyn JAJ, Mao J, Bittner EA (2019) Opioid tolerance in critical illness. N Engl J Med. 380(4):365-378.
Mitchinson AR, Kim HM, Rosenberg JM et al. (2007) Acute postoperative pain management using massage as an adjuvant therapy: A randomized trial. Arch Surg. 142:1158–1167.
Nadeson R, Tucker A, Bajunaki E, Goodchild CS (2002) Potentiation by ketamine of fentanyl antinociception. I. An experimental study in rats showing that ketamine administered by non-spinal routes targets spinal cord antinociceptive systems. Br J Anaesth. 88(5):685-691.
Niraj G, Kellar A, Fox AJ (2009) Application of the transversus abdominis plane block in the intensive care unit. Anaesth Intensive Care. 37(4):650-652.
Nordness MF, Hayhurst CJ, Pandharipande P (2021) Current perspectives on the assessment and management of pain in the intensive care unit. J Pain Res. 14:1733-1744.
Oldenmenger WH, van der Rijt (2018) Feasibility of assessing patient's acceptable pain in a randomized controlled trial on a patient pain education program. Palliat Med. 31(6):553-558.
Pain terms: a list with definitions and notes on usage. Recommended by the IASP Subcommittee on Taxonomy. Pain. 1979. 6(3):249.
Payen JF, Bosson JL, Chanques G et al. (2009) Pain assessment is associated with decreased duration of mechanical ventilation in the intensive care unit: a post hoc analysis of the DOLOREA study. Anesthesiology. 111(6):1308-1316.
Payen JF, Chanques G, Mantz J et al. (2007) Current practices in sedation and analgesia for mechanically ventilated critically ill patients: a prospective multicenter patient-based study. Anesthesiology. 106(4):687-695.
Posa PJ, Singh J, Stollings JL (2020) ICU Liberation. 2nd ed. Mount Prospect, IL: Society of Critical Care Medicine.
Pun BT, Balas MC, Barnes-Daly MA et al. (2019) Caring for critically ill patients with the ABCDEF Bundle: Results of the ICU Liberation Collaborative in Over 15,000 Adults. Crit Care Med. 47(1):3-14.
Puntillo KA, Miaskowski C, Kehrle K et al. (1997) Relationship between behavioral and physiological indicators of pain, critical care patients' self-reports of pain, and opioid administration. Crit Care Med. 25(7):1159-1166.
Puntillo KA, Naidu R (2016) Chronic pain disorders after critical illness and ICU-acquired opioid dependence: two clinical conundrums. Curr Opin Crit Care. 22:506-512.
Rijkenberg S, Stilma W, Bosman RJ et al. (2017) Pain measurement in mechanically ventilated patients after cardiac surgery: Comparison of the Behavioral Pain Scale (BPS) and the Critical-Care Pain Observation Tool (CPOT). J Cardiothorac Vasc Anesth. 31(4):1227-1234.
Robleda G, Roche-Campo F, Sendra MÀ et al. (2016) Fentanyl as pre-emptive treatment of pain associated with turning mechanically ventilated patients: a randomized controlled feasibility study. Intensive Care Med. 42(2):183-191.
Sessler CN, Grap MJ, Ramsay MA (2008) Evaluating and monitoring analgesia and sedation in the intensive care unit. Crit Care. 12 Suppl 3:S2.
Shiva Shahiri T, Richebe P, Richard-Lalonde M et al. (2022) Description of the validity of the Analgesia Nociception Index (ANI) and Nociception Level Index (NOL) for nociception assessment in anesthetized patients undergoing surgery: a systematized review. J Clin Monit Comput. 36(3):623-635.
Shiva Shahiri T, Richebe P, Richard-Lalonde M et al. (2020) Exploration of the Nociception Level (NOL™) Index for Pain Assessment during Endotracheal Suctioning in Mechanically Ventilated Patients in the Intensive Care Unit: An Observational and Feasibility Study. Pain Manag Nurs. 21(5):428-434.
Siffleet J, Young J, Nikoletti S, Shaw T (2007) Patients' self-report of procedural pain in the intensive care unit. J Clin Nurs. 16(11):2142-2148.
Ventafridda V, Saita L, Ripamonti C, De Conno F (1985) WHO guidelines for the use of analgesics in cancer pain. Int J Tissue React. 7(1):93-96.
Warden V, Hurley AC, Volicer L (2003) Development and psychometric evaluation of the pain assessment in advanced dementia (PAINAD) scale. J Am Med Dir Assoc. 4(1):9-15.
Wick EC, Grant MC, Wu CL (2017) Postoperative multimodal analgesia pain management with nonopioid analgesics and techniques: a review. JAMA Surg. 152(7):691-697.
Williamson A, Hoggart B (2005) Pain: a review of three commonly used pain rating scales. J Clin Nurs. 14(7):798-804.
Yamashita A, Yamasaki M, Matsuyama H et al. (2017) Risk factors and prognosis of pain events during mechanical ventilation: a retrospective study. J Intensive Care. 5:17.